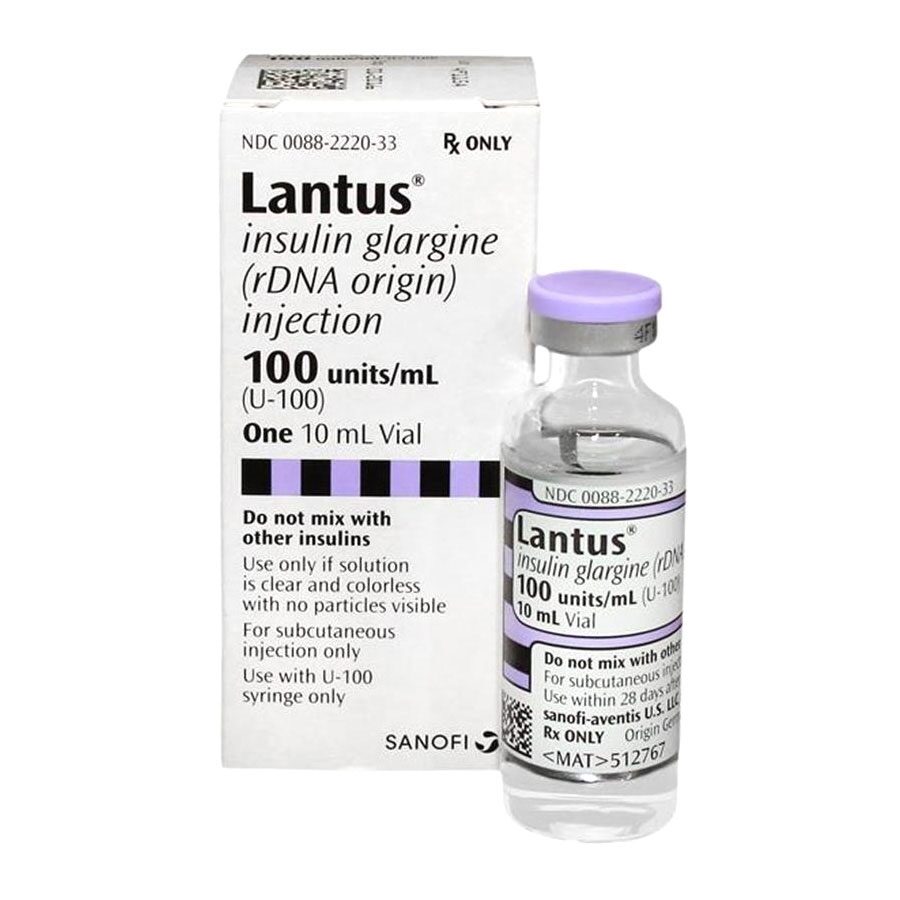
Lantus is long-lasting insulin used to treat adults and children (ages six and older) with type 1 and type 2 diabetes manage high blood glucose levels.
Note: Lantus is not approved to treat insulin ketoacidosis, a common complication among people with diabetes.
Lantus contains insulin glargine, which is long-acting insulin administered through injection under the skin (subcutaneously).
It is typically prescribed to patients in two forms:
In 10-milliliter vials that contain 100 units of insulin glargine. The vials are used with syringes (not included with medication). It is also available in SoloStar Pens.
A disposable single-patient-use prefilled insulin pen contains a 3 ml solution with 100 units of insulin per mL.
Warnings and Precautions
Patients using Lantus vials must never re-use or share needles or syringes with another person. Sharing poses a risk for the transmission of blood-borne pathogens.
Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in insulin strength, manufacturer, type, or method of administration may affect glycemic control and predispose to hypoglycemia or hyperglycemia. These changes should be made cautiously and only under close medical supervision, and the frequency of blood glucose monitoring should be increased. For patients with type 2 diabetes, dosage adjustments of concomitant oral and anti-diabetic products may be needed.
Hypoglycemia
Hypoglycemia is the most common adverse reaction associated with insulin, including Lantus. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
Hypoglycemia can happen suddenly, and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, patients with diabetic nerve disease, patients using medications that block the sympathetic nervous system (e.g., beta-blockers), or in patients who experience recurrent hypoglycemia.
Medication Errors
Accidental mix-ups among insulin products, particularly between long-acting insulins and rapid-acting insulins, have been reported. Instruct patients to check the insulin label before each injection to avoid medication errors between Lantus and other insulins. It is easy to confuse Lantus Solostar pens with Apidra’s Solostar pens. Ensure you are ordering the proper prescription with obtaining your medication.
Hypersensitivity and Allergic Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including Lantus. If hypersensitivity reactions occur, discontinue Lantus; treat per standard of care and monitor until symptoms and signs resolve. Lantus is contraindicated in patients who have had hypersensitivity reactions to insulin glargine or one of the excipients.
Hypokalemia
All insulin products, including Lantus, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia, if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
Fluid Retention and Heart Failure
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR) gamma agonists, can cause dose-related fluid retention, mainly when combined with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including Lantus, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered
Usage
Lantus is administered subcutaneously (under the skin) once per day. Insulin dosage is determined based on factors such as lifestyle, health, and blood glucose levels.
While taking Lantus, blood glucose levels should be tested often and result shared with your doctor. Do not make any adjustments to your insulin or dose requirements without consulting your doctor first.
Do not mix or dilute Lantus with other insulin or solutions as this may cause the insulin to not work as intended or affect blood glucose control which can be severe.
Always check the vial or carton label before use to ensure you are using the correct medication.
Only use Lantus if it appears clear and colorless.
Side Effects
Side effects associated with Lantus can range from mild to severe. The side effects listed below do not include all possible side effects.
The Food & Drug Administration (FDA) tracks side effects associated with approved drugs, tracking through several websites.
Common side effects associated with Lantus include injection site reactions (itchiness, redness, pain, tenderness around injection area), lipodystrophy, rashes, itchy skin, edema (swelling), weight gain, respiratory injections, hypoglycemia (low blood sugar levels).
These side effects typically go away after a few days or weeks. If they become more severe, you should consult your doctor or pharmacist.
Other common side effects from Lantus patients include pain, redness, swelling, itching, thickening of the skin at the injection site(s).
These side effects are typically temporary and go away within a few days or weeks.
Lantus may cause serious side effects, such as severe allergic reactions, and can even be fatal. If you experience any following, seek medical attention immediately: full body rash, difficulty breathing, rapid heartbeat, sweating, swelling of the face, tongue, or throat, shortness of breath, extreme drowsiness, dizziness, or confusion.
This is not a complete list of side effects. Other drugs may interact with Lantus. Discuss side effects and risks with your doctor.
Serious side effects associated with Lantus aren’t common but can occur. These side effects & symptoms include Hypokalemia (low potassium levels). The symptoms of Hypokalemia include abnormal heart rhythm, fatigue, weakness, muscle cramping, paralysis (loss of movement in a body part), and respiratory failure.



Reviews
There are no reviews yet.